Is It Safe To Use Tap Water To Clean Nebulizer
| Nebulizer | |
|---|---|
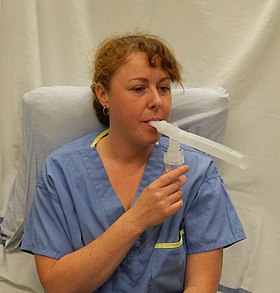 A hospital nebulizer setup | |
| Specialty | pulmonology |
| [edit on Wikidata] | |
In medicine, a nebulizer (American English)[1] or nebuliser (British English)[2] is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, cystic fibrosis, COPD and other respiratory diseases or disorders. They use oxygen, compressed air or ultrasonic power to break up solutions and suspensions into pocket-sized aerosol droplets that are inhaled from the mouthpiece of the device. An aerosol is a mixture of gas and solid or liquid particles.
Medical uses [edit]
Another form of nebulization
Guidelines [edit]
Diverse asthma guidelines, such as the Global Initiative for Asthma Guidelines [GINA], the British Guidelines on the management of Asthma, The Canadian Pediatric Asthma Consensus Guidelines, and United states of america Guidelines for Diagnosis and Treatment of Asthma each recommend metered dose inhalers in identify of nebulizer-delivered therapies.[three] The European Respiratory Society acknowledge that although nebulizers are used in hospitals and at dwelling house they suggest much of this use may not be evidence-based.[iv]
Effectiveness [edit]
Contempo evidence shows that nebulizers are no more than effective than metered-dose inhalers (MDIs) with spacers.[5] An MDI with a spacer may offer advantages to children who accept acute asthma.[3] [vi] [5] Those findings refer specifically to the treatment of asthma and not to the efficacy of nebulisers more often than not, as for COPD for example.[5] For COPD, peculiarly when assessing exacerbations or lung attacks, there is no testify to indicate that MDI (with a spacer) delivered medicine is more effective than administration of the same medicine with a nebulizer.[7]
The European Respiratory Lodge highlighted a adventure relating to droplet size reproducibility caused by selling nebulizer devices separately from nebulized solution. They found this practice could vary droplet size ten-fold or more than by changing from an inefficient nebulizer system to a highly efficient i.[iv] [5] 2 advantages attributed to nebulizers, compared to MDIs with spacers (inhalers), are their ability to evangelize larger dosages at a faster charge per unit, specially in acute asthma; however, recent data suggests actual lung deposition rates are the same. In addition, another trial establish that a MDI (with spacer) had a lower required dose for clinical result compared to a nebulizer (see Clark, et al. other references).[3]
Beyond use in chronic lung illness, nebulizers may besides be used to treat astute bug similar the inhalation of toxic substances. One such example is the handling of inhalation of toxic hydrofluoric acid (HF) vapors.[8] Calcium gluconate is a first-line treatment for HF exposure to the pare. By using a nebulizer, calcium gluconate is delivered to the lungs as an aerosol to counteract the toxicity of inhaled HF vapors.
Aerosol deposition [edit]
The lung deposition characteristics and efficacy of an aerosol depend largely on the particle or droplet size. By and large, the smaller the particle the greater its chance of peripheral penetration and retention. Nonetheless, for very fine particles below 0.5 μm in bore there is a chance of avoiding degradation altogether and being exhaled. In 1966 the Task Group on Lung Dynamics, concerned mainly with the hazards of inhalation of environmental toxins, proposed a model for deposition of particles in the lung. This suggested that particles of more than 10 μm in diameter are most probable to deposit in the oral cavity and throat, for those of 5–x μm diameter a transition from mouth to airway deposition occurs, and particles smaller than 5 μm in bore deposit more oft in the lower airways and are appropriate for pharmaceutical aerosols.[nine]
Types of nebulizers [edit]

A vial of 0.5% albuterol sulfate inhalation solution for nebulizing
Pneumatic [edit]
Jet nebulizer [edit]
The most commonly used nebulizers are jet nebulizers, which are as well called "atomizers".[10] Jet nebulizers are continued past tubing to a supply of compressed gas, unremarkably compressed air or oxygen to catamenia at loftier velocity through a liquid medicine to turn it into an aerosol that is inhaled by the patient. Currently there seems to be a tendency amongst physicians to adopt prescription of a pressurized Metered Dose Inhaler (pMDI) for their patients, instead of a jet nebulizer that generates a lot more than noise (oftentimes 60 dB during use) and is less portable due to a greater weight. Withal, jet nebulizers are commonly used in hospitals for patients who have difficulty using inhalers, such every bit in serious cases of respiratory illness, or astringent asthma attacks.[11] The primary advantage of the jet nebulizer is related to its low operational cost. If the patient needs to inhale medicine on a daily ground the use of a pMDI can exist rather expensive. Today several manufacturers have besides managed to lower the weight of the jet nebulizer to 635 grams (22.4 oz), and thereby started to label it as a portable device. Compared to all the competing inhalers and nebulizers, the noise and heavy weight is yet the biggest draw back of the jet nebulizer.[12]
Mechanical [edit]
Soft mist inhaler [edit]
The medical company Boehringer Ingelheim also invented a device named Respimat Soft Mist Inhaler in 1997. This new technology provides a metered dose to the user, as the liquid bottom of the inhaler is rotated clockwise 180 degrees by mitt, adding a build upward tension into a leap around the flexible liquid container. When the user activates the lesser of the inhaler, the free energy from the spring is released and imposes pressure on the flexible liquid container, causing liquid to spray out of 2 nozzles, thus forming a soft mist to be inhaled. The device features no gas propellant and no need for battery/power to operate. The average droplet size in the mist was measured to 5.8 micrometers, which could indicate some potential efficiency problems for the inhaled medicine to reach the lungs. Subsequent trials accept proven this was not the case. Due to the very depression velocity of the mist, the Soft Mist Inhaler in fact has a college efficiency compared to a conventional pMDI.[13] In 2000, arguments were launched towards the European Respiratory Order (ERS) to clarify/expand their definition of a nebulizer, as the new Soft Mist Inhaler in technical terms both could exist classified every bit a "hand driven nebulizer" and a "mitt driven pMDI".[14]
Electrical [edit]
Ultrasonic wave nebulizer [edit]
Ultrasonic wave nebulizers were invented in 1965[15] as a new type of portable nebulizer. The engineering inside an ultrasonic wave nebulizer is to have an electronic oscillator generate a high frequency ultrasonic wave, which causes the mechanical vibration of a piezoelectric element. This vibrating element is in contact with a liquid reservoir and its loftier frequency vibration is sufficient to produce a vapor mist.[16] Every bit they create aerosols from ultrasonic vibration instead of using a heavy air compressor, they just accept a weight effectually 170 grams (6.0 oz). Another reward is that the ultrasonic vibration is near silent. Examples of these more than modern blazon of nebulizers are: Omron NE-U17 and Beurer Nebulizer IH30.[17]
Vibrating mesh technology [edit]
A new significant innovation was made in the nebulizer market effectually 2005, with creation of the ultrasonic Vibrating Mesh Technology (VMT). With this applied science a mesh/membrane with yard–7000 light amplification by stimulated emission of radiation drilled holes vibrates at the acme of the liquid reservoir, and thereby pressures out a mist of very fine droplets through the holes. This applied science is more efficient than having a vibrating piezoelectric element at the bottom of the liquid reservoir, and thereby shorter treatment times are also accomplished. The erstwhile problems constitute with the ultrasonic wave nebulizer, having too much liquid waste and undesired heating of the medical liquid, have as well been solved by the new vibrating mesh nebulizers. Available VMT nebulizers include: Pari eFlow,[18] Respironics i-Beak,[xix] Beurer Nebulizer IH50,[20] and Aerogen Aeroneb.[21] As the price of the ultrasonic VMT nebulizers is college than models using previous technologies, most manufacturers continue to also sell the classic jet nebulizers.[22]
Utilize and attachments [edit]
Nebulizers have their medicine in the course of a liquid solution, which is often loaded into the device upon apply. Corticosteroids and bronchodilators such as salbutamol (albuterol USAN) are ofttimes used, and sometimes in combination with ipratropium. The reason these pharmaceuticals are inhaled instead of ingested is in order to target their effect to the respiratory tract, which speeds onset of action of the medicine and reduces side effects, compared to other alternative intake routes.[11]
Usually, the aerosolized medicine is inhaled through a tube-like mouthpiece, similar to that of an inhaler. The mouthpiece, however, is sometimes replaced with a confront mask, similar to that used for inhaled anesthesia, for ease of use with young children or the elderly. Pediatric masks are frequently shaped like animals such as fish, dogs or dragons to make children less resistant to nebulizer treatments. Many nebulizer manufacturers also offer pacifier attachments for infants and toddlers. But mouthpieces are preferable if patients are able to use them since face-masks result in reduced lung delivery because of aerosol losses in the nose.[10]
Later on use with corticosteroid, information technology is theoretically possible for patients to develop a yeast infection in the mouth (thrush) or hoarseness of vox (dysphonia), although these conditions are clinically very rare. To avert these agin effects, some clinicians advise that the person who used the nebulizer should rinse his or her mouth. This is not true for bronchodilators; nevertheless, patients may notwithstanding wish to rinse their mouths due to the unpleasant sense of taste of some bronchodilating drugs.
History [edit]
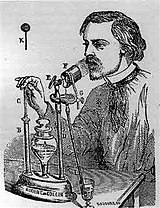
Sales-Girons pressurized nebulizer from 1858
The first "powered" or pressurized inhaler was invented in French republic by Sales-Girons in 1858.[23] This device used pressure level to disintegrate the liquid medication. The pump handle is operated similar a bike pump. When the pump is pulled up, it draws liquid from the reservoir, and upon the force of the user'south hand, the liquid is pressurized through an atomizer, to be sprayed out for inhalation well-nigh the user'southward rima oris.[24]
In 1864, the start steam-driven nebulizer was invented in Federal republic of germany. This inhaler, known as "Siegle's steam spray inhaler", used the Venturi principle to disintegrate liquid medication, and this was the very get-go of nebulizer therapy. The importance of droplet size was non even so understood, so the efficacy of this first device was unfortunately mediocre for many of the medical compounds. The Siegle steam spray inhaler consisted of a spirit burner, which boiled h2o in the reservoir into steam that could and so flow across the summit and into a tube suspended in the pharmaceutical solution. The passage of steam drew the medicine into the vapor, and the patient inhaled this vapor through a mouthpiece made of drinking glass.[25]
The first pneumatic nebulizer fed from an electrically driven gas (air) compressor was invented in the 1930s and called a Pneumostat. With this device, a medical liquid (typically epinephrine chloride, used as a bronchial muscle relaxant to reverse constriction).[26] Every bit an alternative to the expensive electrical nebulizer, many people in the 1930s continued to use the much more than uncomplicated and cheap hand-driven nebulizer, known as the Parke-Davis Glaseptic.[27]
In 1956, a applied science competing against the nebulizer was launched by Riker Laboratories (3M), in the class of pressurized metered-dose inhalers, with Medihaler-iso (isoprenaline) and Medihaler-epi (epinephrine) as the ii starting time products.[28] In these devices, the drug is common cold-fill and delivered in verbal doses through some special metering valves, driven by a gas propellant applied science (i.eastward. Freon or a less environmentally damaging HFA).[23]
In 1964, a new type of electronic nebulizer was introduced: the "ultrasonic wave nebulizer".[29] Today the nebulizing technology is not only used for medical purposes. Ultrasonic moving ridge nebulizers are as well used in humidifiers, to spray out water aerosols to moisten dry air in buildings.[xvi]
Some of the first models of electronic cigarettes featured an ultrasonic wave nebulizer (having a piezoelectric element vibrating and creating high-frequency ultrasound waves, to cause vibration and atomization of liquid nicotine) in combination with a vapouriser (built as a spray nozzle with an electrical heating element).[xxx] The almost mutual blazon of electronic cigarettes currently sold, yet, omit the ultrasonic moving ridge nebulizer, as it was non found to be efficient enough for this kind of device. Instead, the electronic cigarettes now use an electric vaporizer, either in directly contact with the absorbent material in the "impregnated atomizer," or in combination with the nebulization technology related to a "spraying jet atomizer" (in the class of liquid droplets being out-sprayed by a high-speed air stream, that passes through some small-scale venturi injection channels, drilled in a material absorbed with nicotine liquid).[31]
See too [edit]
- Inhaler
- Humidifier
- Vaporizer
- Listing of medical inhalants
- Spray canteen
References [edit]
- ^ Medical Lexicon. "Nebulizer". Retrieved 2010-11-01 .
- ^ British Spelling of Nebulizer Medical Lexicon. "Definition". Archived from the original on 2022-07-01. Retrieved 2010-11-01 .
- ^ a b c Clark NM, Houle C, Partridge MR, Leo HL, Paton JY (2010). "The puzzle of continued use of nebulized therapy by those with asthma". Chron Respir Dis. seven (1): 3–7. doi:10.1177/1479972309357496. PMID 20103617.
- ^ a b Boe J, Dennis JH, O'Driscoll BR, et al. (July 2001). "European Respiratory Social club Guidelines on the use of nebulizers". Eur. Respir. J. 18 (i): 228–42. doi:10.1183/09031936.01.00220001. PMID 11510796.
- ^ a b c d Cates, Christopher J.; Welsh, Emma J.; Rowe, Brian H. (2013-09-xiii). "Holding chambers (spacers) versus nebulisers for beta-agonist handling of acute asthma". The Cochrane Database of Systematic Reviews (9): CD000052. doi:ten.1002/14651858.CD000052.pub3. ISSN 1469-493X. PMC7032675. PMID 24037768.
- ^ Epling J, Chang MH (January 2003). "Are metered-dose inhalers with holding chambers better than nebulizers for treating acute asthma?". Am Fam Md. 67 (1): 62–four. PMID 12537167.
- ^ van Geffen, Wouter H.; Douma, Westward. R.; Slebos, Dirk Jan; Kerstjens, Huib A. M. (2016-08-29). "Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD" (PDF). The Cochrane Database of Systematic Reviews. 2016 (8): CD011826. doi:x.1002/14651858.CD011826.pub2. ISSN 1469-493X. PMC8487315. PMID 27569680.
- ^ Kono, K (2000). "Successful treatments of lung injury and skin burn due to hydrofluoric acid exposure". International Athenaeum of Occupational and Environmental Health. 73 Suppl: S93-7. doi:10.1007/pl00014634. PMID 10968568. S2CID 37322396.
- ^ The scientific discipline of nebulised drug commitment, p6
- ^ a b Finlay, Westward.H. (2001). The Mechanics of Inhaled Pharmaceutical Aerosols: An Introduction. Academic Press.
- ^ a b Hickey, A.J. (2004). Pharmaceutical Inhalation Droplets Engineering science (2d ed.). New York: Marcel Dekker. ISBN9780824742539.
- ^ J. Jendle; B. Eastward. Karlberg; J. Persliden; L. Franzen; Yard. Jr Arborelius (Fall 1995). "Delivery and retention of an insulin droplets produced by a new jet nebulizer". Journal of Droplets Medicine. 8 (iii): 243–254. doi:10.1089/jam.1995.eight.243. PMID 10155650.
- ^ Boehringer Ingelheim (2003). "How information technology works: Respimat Soft Mist Inhaler". Archived from the original on 2007-05-27. Retrieved 2005-08-16 .
- ^ Denyer J, et al. (2000). "New liquid drug aerosol devices for inhalation therapy". Eur Respir Rev. x: 187–191.
- ^ Us patent 3243122, Snaper, Alvin A., "Ultrasonic Spray Apparatus", published 29 March 1966
- ^ a b BOGA Gmbh. "Operating principle of ultrasonic humidifier". Archived from the original on 2022-11-fourteen. Retrieved 2010-04-05 .
- ^ Knoch, Thou.; Finlay, Westward.H. (2002). "Ch. 71 Nebuliser Technologies". In Rathbone; Hadgraft; Roberts (eds.). Modified-Release Drug Delivery Technology. Marcel Dekker. pp. 849–856.
- ^ PARI Pharma (2008). "Leading aerosol therapies worldwide, delivery with eFlow". Archived from the original on 2022-03-28. Retrieved 2010-04-09 .
- ^ Philips Respironics (2010). "Active Droplets Delivery, The I-bill and Vibrating Mesh Technology". Archived from the original on 2022-08-04. Retrieved 2010-04-09 .
- ^ Beurer (2015). "Product details of IH50 nebulizer, with a vibrating membrane". Retrieved 2015-04-21 .
- ^ Aerogen (2009). "Micropump nebulizers, Aeroneb, Vibrating Mesh Engineering science". Archived from the original on 2022-02-03. Retrieved 2010-04-09 .
- ^ Team, Editorial (2019-12-09). "Nebulizer Machine and Its Overview". Index of Sciences . Retrieved 2020-05-25 .
- ^ a b Sanders One thousand (April 2007). "Inhalation therapy: an historical review" (PDF). Prim Care Respir J. 16 (ii): 71–81. doi:ten.3132/pcrj.2007.00017. PMC6634187. PMID 17356785.
- ^ Inhalatorium. "Pressurized inhaler invented by Sales-Girons". Archived from the original on 2022-01-03. Retrieved 2010-04-05 .
- ^ Inhalatorium. "Siegle's steam spray inhaler". Archived from the original on 2004-08-26. Retrieved 2010-04-05 .
- ^ Inhalatorium. "First electrical nebulizer (Pneumostat)". Archived from the original on 2005-02-17. Retrieved 2010-04-05 .
- ^ Inhalatorium. "The manus driven nebulizer "Parke-Davis Glaseptic". Archived from the original on 2004-09-06. Retrieved 2010-04-05 .
- ^ Riker Laboratories (1960-03-16) [1956-03-21]. "Self-propelling pharmaceutical compositions (for a pMDI)". GB patent . Retrieved 24 January 2022.
- ^ Devilbiss Co. (1967-05-17) [1964-02-10]. "Method and appliance for producing aerosols (ultrasonic nebulizer)". GB patent . Retrieved 24 Jan 2022.
- ^ Hon Lik (2004-04-fourteen). "An aerosol electronic cigarette". CN patent . Retrieved 2006-12-27 .
- ^ Hon Lik (2006-05-16). "Emulation aerosol sucker". CN patent . Retrieved 2009-02-11 .
Source: https://en.wikipedia.org/wiki/Nebulizer
Posted by: moodyalwask.blogspot.com


0 Response to "Is It Safe To Use Tap Water To Clean Nebulizer"
Post a Comment